Research
T cells are white blood cells that initiate immune responses that can clear infections and cancers. The decision to respond is made by the integration of signals from many different T cell surface receptors using many intracellular signalling molecules. We use a diverse set of quantitative methods to uncover the mechanisms underlying signal integration and exploit this new information to improve human health.
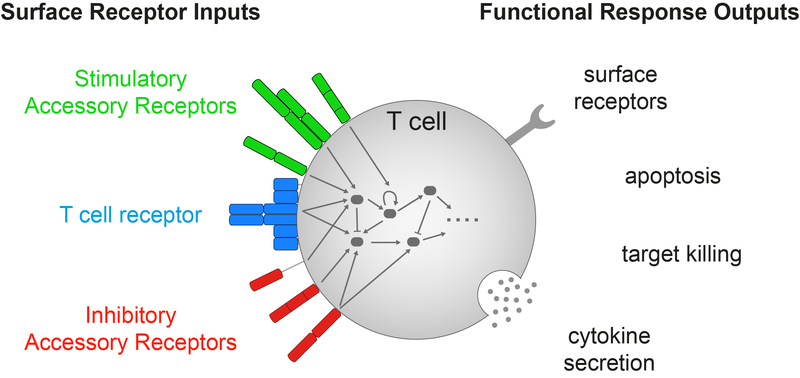
T cell antigen sensitivity
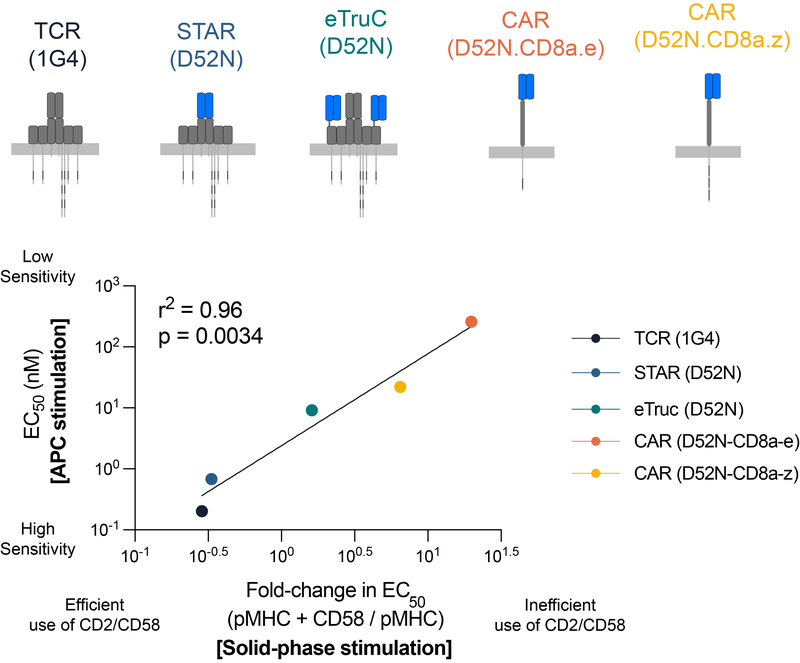
T cells can remarkably recognise a single antigen on the surface of other cells. This remarkable antigen sensitivity is thought to be important because pathogens and cancers evolve to reduce the amount of antigen they present. Moreover, many therapies are being developed to re-direct T-cells to kill cancer cells by making them express a synthetic antigen receptor that can specifically recognise cancer cells. This therapeutic approach is very effective for some cancers that express high levels of antigens. However, many patients relapse when cancer cells emerge that have lower antigen levels on their surface. We now know that these cancer cells are not recognized because unlike the native TCR, these synthetic receptors have a profound antigen sensitivity defect that requires high levels of antigen to activate T-cells. There is now an urgent need to increase the sensitivity of synthetic receptors to prevent relapses.
We have been working to understand the contribution of co-stimulation and co-inhibition receptors to T cell antigen sensitivity mediated by the native TCR and a variety of synthetic antigen receptors (Patel et al (2024) EMBO J; Burton et al (2023) PNAS)
T cell antigen discrimination
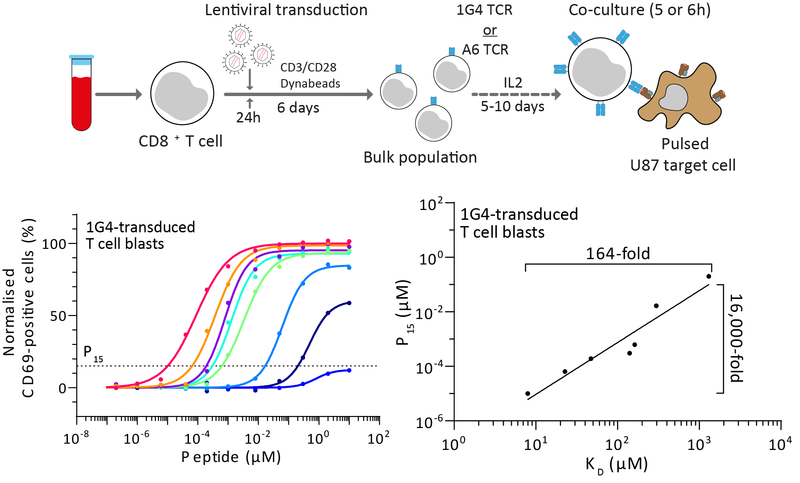
T cells continuously patrol the body in search of antigens derived from pathogens and cancers. They do this using their T cell receptors (TCRs) that can bind to peptide antigens displayed on major-histocompatibility-complexes (pMHCs) on the surfaces of nearly all cells in the body. Using mathematical modelling and experiments, it is now clear that a single T cell can respond to >100,000 different pMHCs. This high level of peptide cross-reactivity is thought to be essential for the relatively small number of T cells to recognise the large number of potential foreign peptides. Despite their high cross-reactivity, T cells normally do not respond to self peptides (self pMHC). We have been studying the molecular mechanisms that enable T cells to discriminate between lower-affinity self and higher-affinity non-self pMHCs.
We have developed accurate methods to measure TCR/pMHC affinities that enabled us to precisely quantify the discriminatory power of the TCR, and to understand the contribution of different surface receptors to this process (Pettmann et al (2021) eLife; Pettmann et al (2023) EMBO J).
Tools to study signal integration by surface receptors
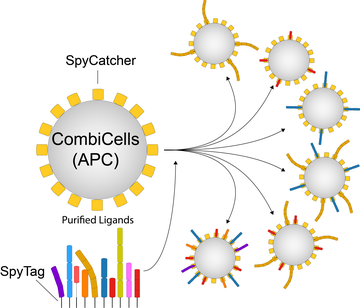
A major limitation of studying surface receptors on T cells and other immune cells lies in our inability to independently control their ligands on the surfaces of target cells. In contrast to surface receptors that recognise ligands in solution (e.g. GPCRs, RTKs, Cytokine receptors), our understanding of receptor/ligand interactions at cell/cell interfaces has been hampered by the inability to control the combinations and concentrations of ligands on cell surfaces. This is particularly important for T cells that must scan nearly all cells in the body whether normal or abnormal and therefore, experience surfaces with diverse concentrations/combinations of ligands to their accessory receptors.
We have developed a new method for the combinatorial display and titration of ligands directly on the cell surface (CombiCells). We recently used this platform to study the contribution of co-stimulation/co-inhibition receptors on T cell antigen sensitivity mediated by TCRs, CARs, and BiTEs (Patel et al (2024) EMBO J).
Receptor triggering by kinetic-segregation
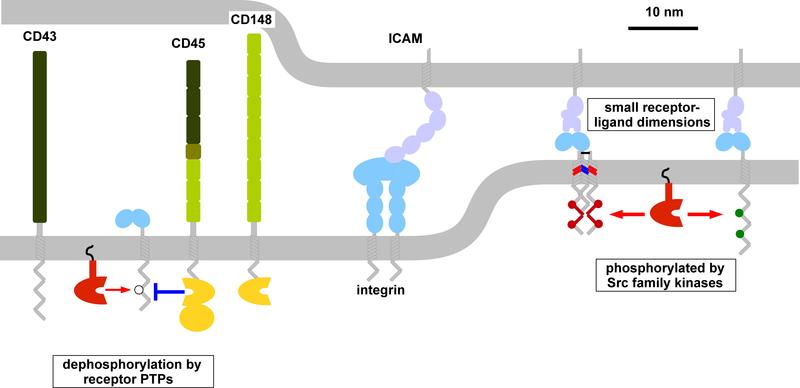
The function of immune cells is controlled by an array of cell-surface receptors that lack sequence homology. However, many of these receptors share common features, including the fact that their signaling involves phosphorylation of their unstructured cytoplasmic domains by extrinsic tyrosine kinases. These non-catalytic tyrosine-phosphorylated receptors (NTRs) share a number of other features, including small extracellular size and optimal stimulation by surface-associated ligands.
We have hypothesised that NTRs share the same kinetic-segregation mechanism of receptor triggering, which is the process by which extracellular ligand binding induces intracellular signalling. The model proposes that ligand binding induces segregation of the large phosphatases CD45 and CD148 from NTRs increasing the net kinase activity. This mechanism of triggering has several features uniquely well suited for their role in immune recognition of infection and cancer, including the ability to co-localise at cell-cell interfaces facilitating signal integration (Dushek et al (2012) Immunological Reviews).


