Research Highlights
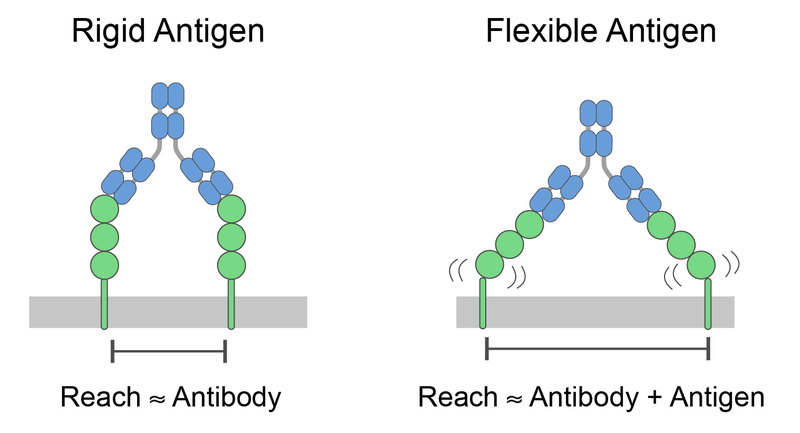
The molecular reach of antibodies crucially underpins their viral neutralisation capacity
Huhn A, Nissley D, Wilson DB, Kutuzov M, Donat R, Tan TK, Zhang Y, Barton MI, Liu C, Dejnirattisai W, Supasa P, Mongkolsapaya J, Townsend A, James W, Screaton G, van der Merwe PA, Deane CM, Isaacson SA, Dushek O
Nature Communications (2025)
- The function of antibodies relies on high-affinity binding to their targets, which is often achieved through bivalent binding.
- Although bivalent binding is important, we lack methods to mechanistically analyse bivalent binding of soluble antibodies to surface antigen.
- Here, we developed and validated a particle-based model to analyse bivalent binding.
- The model provides the usual monovalent binding kinetics (kon, koff, KD) and new bivalent parameters, including the molecular reach: the maximum antigen separation that supports bivalent binding.
- We found large variations in the molecular reach (22-46 nm) for antibodies within an isotype class binding the same antigen.
- We show that the molecular reach exceeded the physical size of the antibody (~15 nm) because of the contribution of the antigen.
- The molecular reach was the best single-parameter predictor of viral neutralisation.
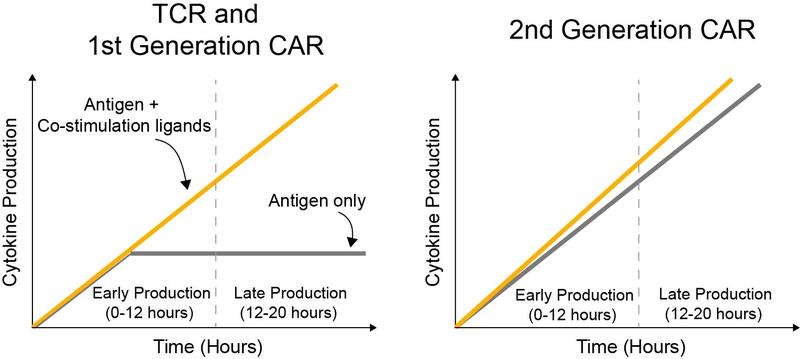
Regulation of temporal cytokine production by co-stimulation receptors in TCR-T cells is lost in CAR-T cells
Patel A, Kutuzov MA, Dustin ML, van der Merwe PA, Dushek O
Immunotherapy Advances (2024)
- Immune therapies using chimeric antigen receptors (CAR)-T cells often lead to toxicities that have been traced to excessive cytokine production.
- We compared the temporal kinetics of cytokine production by 1st and 2nd generation CAR-T cells and TCR-T cells.
- We found that TCR-T cells and 1st generation CAR-T cells stopped producing cytokines unless ligands to co-stimulation receptors were presented alongside antigen.
- In contrast, 2nd generation CAR-T cells continued to produce cytokines in response to antigen alone without requiring ligands to co-stimulation receptors.
- Losing the requirement for costimulation for sustained cytokine production may contribute to the effectiveness and/or toxicity of 2nd-generation CAR-T-cell therapy.

Immune therapies using chimeric antigen receptors (CAR)-T cells have a major defect in antigen sensitivity and this leads to cancer relapse.
- We have discovered methods to improve the sensitivity of CAR-T cells.
- We secured venture capital seed funding to spin-out a company to improve these therapies.
- MatchBio Ltd has been operational as of January 2024.
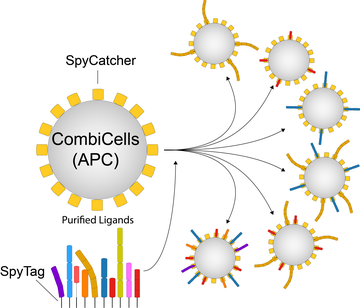
Using CombiCells, a platform enabling titration and combinatorial display of cell surface ligands, to study T cell antigen sensitivity by TCRs, CARs, and BiTEs.
Patel A, Andre V, Eguiguren SB, Barton MI, Denham EM, Pettmann J, Morch AM, Kutuzov MA, Siller-Farfan JA, Dustin ML, van der Merwe PA, Dushek O
EMBO J (2024)
- Our ability to study cell-cell recognition has been limited by our inability to independently control cell surface ligands.
- Here, we expressed the protein SpyCatcher, which forms a covalent bond with SpyTag, on the cell surface.
- By adding any combination and concentration of purified ligands fused to Spytag, we can produce cells with desired ligand combinations/concentrations within minutes.
- This platform has many applications, including studying the basic mechanism of cell-cell recognition and evaluating the performance of immunotherapies, such as BiTEs and CARs.
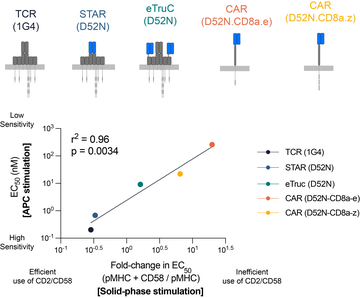
Inefficient exploitation of accessory receptors reduces the sensitivity of chimeric antigen receptors
Burton J, Siller-Farfan JA, Pettmann J, Salzer B, Kutuzov M, van der Merwe PA, Dushek O
PNAS (2023)
-
The TCR uses diverse mechanisms to achieve its remarkable single pMHC ligand sensitivity (Siller-Farfan & Dushek (2018) Immunological Reviews).
-
However, Chimeric Antigen Receptors (CARs) have a profound sensitivity defect requiring >100-fold more antigen to activate T cells and this is thought to contribute to cancer relapse in patients.
-
By systematically studying the impact of different co-stimulation receptors on TCR and CAR antigen sensitivity, we identified that CARs fail to efficiently exploit adhesion receptors to enhance their antigen sensitivity.
-
We show that the ability of a variety of synthetic antigen receptors to exploit the adhesion receptor CD2 and LFA-1 can predict their antigen sensitivity.
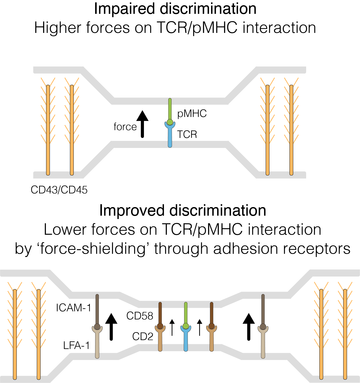
Mechanical forces impair antigen discrimination by reducing differences in T cell receptor off-rates
Pettmann J, Awada L, Bartosz R, Huhn A, Faour S, Kutuzov M, Limozin L, Weikl TR, van der Merwe PA, Robert P, Dushek O
EMBO Journal (2023), see associated News & Views
- The T cell antigen receptor (TCR) needs to discriminate between lower-affinity self pMHCs and higher-affinity foreign pMHCs.
- Previous work suggested that mechanical forces may impact the ability of T cells to discriminate pMHC antigens.
- Here, we used a cell-free system to study how mechanical forces impact the TCR/pMHC off-rate and find that mechanical pulling forces decrease differences in off-rates impairing antigen discrimination.
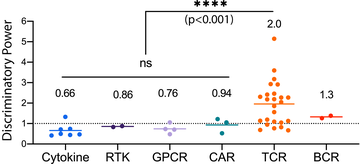
The discriminatory power of the T cell receptor
*Pettmann J, *Huhn A, *Abu Shah E, Kutuzov MA, Wilson DB, Dustin ML, Davis SJ, van der Merwe PA, Dushek O
eLife (2021), see associated Press Release.
- The large community of T cell immunologists, including ourselves, have focused on explaining the apparent near-perfect ability of T cells to discriminate antigen based on the TCR/pMHC binding affinity.
-
However, we discovered that the foundational data on antigen discrimination used by the community contained artefacts in their affinity measurements
-
In this work, we developed a method to accurately measure TCR/pMHC affinities and used it to precisely quantify antigen discrimination.
-
We show that the discriminatory power of the TCR, although enhanced compared to conventional surface receptors (e.g. GPCR, RTKs, etc), is imperfect allowing responses even from ultra-low affinity pMHCs.
-
This suggests that T cell mediated autoimmunity is not necessarily a T cell defect but rather a defect in target cells that cause them to abnormally express high levels of self-antigens.


